Evidence Supporting the Safety of Rapid Patient Cooling
Robert B. Schock, Ph.D.
From the Department of Research and Development, The Sid Wolvek Research Center, Life Recovery Systems HD LLC, Kinnelon, NJ USA
As the applications for targeted temperature management are evolving, an increasing body of evidence supports the rationale for more rapid achievement of target therapeutic temperatures. This report examines the safety of a new technique for achieving rapid patient cooling [LRS Technical Report, March 2012).
In the wake of the latest patient care guidelines from the American Heart Association[1], therapeutic hypothermia is quickly gaining acceptance as a standard of care. A growing body of evidence is suggesting that the use of more rapid patient cooling methods is desirable in this application, as this may reduce the side effects of hypothermia induction, such as shivering and metabolic disorders[2]. The use of rapid cooling techniques has been questioned by some on the basis of safety considerations related to high cooling rates and risks of overcooling. These issues are discussed below.
High Cooling Rates
Therapeutic patient cooling has been actively studied for over half a century. One of the more prominent applications has been the use of cooling to prevent brain and other organ injury during prolonged periods of cardiac arrest, as typically occur in open heart surgery. The typical “on-pump†cardiac bypass procedure involves the use of a heart lung machine, which cools the patient by approximately 20C° in 30 minutes[3], a cooling rate of 40C°/hour. This procedure has been safely administered to millions of patients. Complications which result from this invasive method are typically the result of bleeding, embolic episodes, or blood damage.
The most rapid noninvasive cooling method known is ice water immersion. This has been shown to generate a cooling rate of approximately 0.35C°/min (21C°/hour) in hyperthermic awake volunteers[4], with no adverse side effects. A desirable characteristic of the ice water immersion treatment is that shivering is generally suppressed at the lowest water temperatures (~2C°). A second important observation is that the cooling rate during ice water immersion is so rapid that a rectal temperature inaccurately reflects core temperature during the cooling process; an esophageal temperature probe more accurately reflects true core temperature during this rapid cooling[5].
Ice water immersion cooling has also been studied in normothermic anesthetized volunteers who have been cooled to hypothermic levels[6]. In these studies, the subjects were typically cooled from normothermia to 34°C as a result of a 20 minute period of immersion, a cooling rate of 9.7C°/hour. This was approximately six times as fast as the cooling provided by conventional cooling blankets. As in the prior studies of hyperthermic volunteers, this rapid cooling rate did not cause any adverse effects.
Exertional heatstroke is a deadly condition that requires the most aggressive treatment measures. This condition can kill or permanently disable even young, well- conditioned athletes. Ice water immersion is well recognized as the standard of care for treatment of exertional heatstroke, a condition in which rapid, early cooling is critically important to patient recovery[7]. This treatment has been applied in hundreds of well-documented cases, with extremely high cooling rates and a lack of adverse side effects.
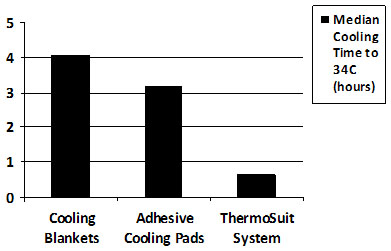
The application of rapid cooling methods for the induction of therapeutic hypothermia has recently seen increased interest. A study of 24 patients treated with the Life Recovery Systems ThermoSuit System, a product which cools with convective ice water immersion, reported patient cooling rates averaging 3.5C°/hour (range, 1.8 to 8.8C°/hour)[8].  The clinical safety profile of the ThermoSuit treatment as reported in this study compares very favorably with that reported in a similar study of 64 patients, in which conventional cooling blankets and the Medivance Arctic Sun (a system that employs adhesive-faced cooling pads) were used; these cooling methods demonstrated cooling rates approximately five times slower than those of the ThermoSuit approach[9]. A comparison of hypothermia induction times reported for these studies is provided in Figure 1.
Another clinical study of therapeutic hypothermia investigated the use of an invasive rapid cooling protocol which included the use of cold intravenous saline infusion and an intravascular cooling catheter[10]. This approach produced a typical cooling rate of 3.6 C°/hour, a rate similar to that of the noninvasive ThermoSuit method. Aside from a 33% incidence of infections that resulted from this invasive approach, there were no clinical complications of the rapid cooling induction. Overall, there were fewer complications in the cooled patients than in a control group of normothermic patients.
Further clinical evidence supporting the safety of rapid patient cooling was provided by a four-year study that showed that even delays of one hour in initiating cooling or 30 minutes in achieving target therapeutic temperatures had a negative impact[11], [12]. These results validated those of a previously published study, which also reported a strong benefit of earlier achievement of target temperature[13].
The advantages of faster achievement of target temperature are most apparent when adjustments are made for patient comorbidities as part of the data analysis; patients who have suffered more severe cerebral injury tend to lose the ability to conserve their own body heat and are more easily cooled[14]. Previous studies which have not made such adjustments have sometimes failed to demonstrate a benefit of faster achievement of target temperature[15], [16].
Numerous laboratory studies have demonstrated benefits of fast, early cooling[17], [18], [19], [20], [21], [22], [23], [24]. Temperature dependent adverse biochemical reactions are inhibited by hypothermia. Early intervention clearly minimizes the time that these reactions have to accumulate adverse byproducts. While additional clinical studies investigating the relationship between cooling speed and outcomes would be helpful, studies conducted thus far support the hypothesis that the use of faster cooling methods is beneficial to patients. Likewise, these studies have shown no significant evidence that the use of faster cooling methods is harmful.
Â
Overcooling
All surface cooling methods have some potential to overcool patients, as has been reported for cooling blankets and the Medivance Arctic Sun device; overcooling by approximately 4C° has been reported to occur with both of these methods9. Humans subjected to ice water immersion have been reported to continue to drop in temperature by an additional 0.56°C to nearly 2°C after being removed from the water5, 6. The ThermoSuit System, which is the only available product which uses ice water immersion to cool patients, anticipates this “afterdrop†and a message on the display instructs the user to purge the water from the suit at 1.5°C above the target temperature. The water is automatically purged if the temperature drops within 1°C of the target. To assure an accurate assessment of core body temperature during rapid cooling, the Instructions for Use of the ThermoSuit System specify the use of esophageal or nasopharyngeal temperature monitoring, as opposed to bladder or rectal temperature monitoring; this further reduces the probability of overcooling. As an additional measure to avoid overcooling, the Instructions for Use recommend that the user immediately remove the patient from the ThermoSuit upon completion of the purge process, and place the patient on a conventional cooling/warming blanket to assure that the patient remains within the targeted range of temperatures. These approaches have proven successful in the achievement of safe, rapid and accurate cooling of hundreds of patients with the ThermoSuit System.
Conclusions
New evidence supports the clinical value of rapid patient cooling in a number of applications. Cooling by ice water immersion, as provided by the Life Recovery Systems ThermoSuit® System, offers potentially significant clinical benefits. By using an accurate core temperature monitoring method (esophageal or nasopharyngeal monitoring) and feedback-controlled cessation of cooling induction, this simple noninvasive approach is capable of providing safe, rapid, and accurate patient cooling. It offers an important new clinical option for cooling patients where clinically indicated.
FDA-Cleared Indications for The LRS ThermoSuit System:
Temperature reduction in patients where clinically indicated, e.g. in hyperthermic patients.
 CE and Health Canada – Cleared Indications for the LRS ThermoSuit System:
Temperature reduction in patients where clinically indicated, e.g., to induce hypothermia in patients to preserve cardiac and brain function in victims of cardiac arrest, stroke, heart attack, traumatic brain injury.
References
[1] ECC Committee, Subcommittees and Task Forces of the American Heart Association, “2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Careâ€, Circulation 2010;122:S768-S786.
[2] Polderman K and Herold I, “Therapeutic hypothermia and controlled normothermia In the intensive care unit: Practical considerations, side effects, and cooling methodsâ€, Crit Care Med 2009; 37(3): 1101-1120.
[3] Stone G et al, “Do Standard Monitoring Sites Reflect True Brain Temperature When Profound Hypothermia is Rapidly Induced and Reversed?â€, Anesthesiology 1995; 82(2): 344-351.
[4] Proulx CI et al, “Effect of water temperature on cooling efficiency during hyperthermia in humans“, J Appl Physiol 2003;Â 94(4):1317-1323.
[5] Proulx CI et al, “Safe cooling limits from exercise-induced hyperthermiaâ€, Eur J Appl Physiol 2006; 96:434-445.
[6] Plattner, O et al, ” Efficacy of Intraoperative Cooling Methods”, Anesthesiology 1997; 87(5):1089-1095.
[7] Casa DJ, McDermott EC, Le SW et al, “Cold Water Immersion: The Gold Standard for Exertional Heatstroke Treatmentâ€, Exerc Sport Sci Rev 2007, 35; 3:141-149.
[8] Howes D, Ohley W, Dorian P, et al, “Rapid Induction of Therapeutic Hypothermia using Convective-Immersion Surface Cooling: Safety, Efficacy, and Outcomesâ€, Resuscitation 2010 Vol. 81, no .4, 388-392.
[9] Heard KJ, Peberdy MA, Sayre MR et al, “A randomized controlled trial comparing the Arctic Sun to standard cooling for induction of hypothermia after cardiac arrestâ€, Resuscitation 2010 Vol 81, No. 1, 9-14.
[10] Götberg M, Olivecrona GK, Koul S et al, “A Pilot Study of Rapid Cooling by Cold Saline and Endovascular Cooling Before Reperfusion in Patients With ST-Elevation Myocardial Infarctionâ€, Circ Cardiovasc Interv 2010:3;400-407.
[11] Mooney MR, Unger BT, Boland LL et al, “Therapeutic Hypothermia After Out-of-Hospital Cardiac Arrest: Evaluation of a Regional System to Increase Access to Coolingâ€, Circulation 2011, 124:206-214.
[12] Sendelbach S, Hearst MO, Johnson PJ, Unger BT, Mooney MR, “Effects of Variation in Temperature Management on Cerebral Performance Category Scores in Patients Who Received Therapeutic Hypothermia Post Cardiac Arrestâ€. Resuscitation (2012), doi:10.1016/j.resuscitation.2011.12.026
[13] Wolff B et al, “Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrestâ€, International Journal of Cardiology 2009.
[14] Felberg RA et al, “Hypothermia After Cardiac Arrest – Feasibility and Safety of an External Cooling Protocolâ€, Circulation 2001; 104: 1799-1804.
[15] Haugk M et al, “Relationship between time to target temperature and outcome in patients treated with therapeutic hypothermia after cardiac arrestâ€, Critical Care 2011, 15:R101.
[16] Sawyer KN et al, “The Impact of Time to Target Temperature on Survival to Hospital Discharge for Patients Undergoing a Comprehensive Postresuscitation Program Following Out-of-Hospital Cardiac Arrestâ€, Circulation 2011 (Abstract); 124: A7.
[17] Busto R, Dietrich WD, Globus MY, Ginsberg MD, “Postischemic moderate hypothermia inhibits CA1 hippocampal ischemic neuronal injuryâ€, Neurosci Lett. 1989 Jul 3; 101(3): 299-304.
[18] Kuboyama K et al, “Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized studyâ€, Crit Care Med 1993; 211348-58.
[19] Janata A, Weihs W, Bayegan K et al, “Therapeutic hypothermia with a novel surface cooling device improves neurologic outcome after prolonged cardiac arrest in swine“, Crit Care Med 2008 ;36(3): 895-902.
[20] Zhao H, Steinberg G, “Limited Therapeutic Time Windows of Mild-to-Moderate Hypothermia in a Focal Ischemia Model in Ratâ€, Stroke Research and Treatment 2011, Article ID 131834.
[21] Markgraf CG, Clifton GL, Moody MR, “Treatment window for hypothermia in brain injuryâ€, J Neurosurg. 2001; 1995(6):979-83.
[22] Carroll M, Beek O, “Protection against hippocampal CA1 cell loss by post-ischemic hypothermia is dependent on delay of initiation and durationâ€, Metab Brain Dis. 1992;7(1):45-50.